Early Development
Studying how the fertilised egg gives rise to all of the cells in the human body
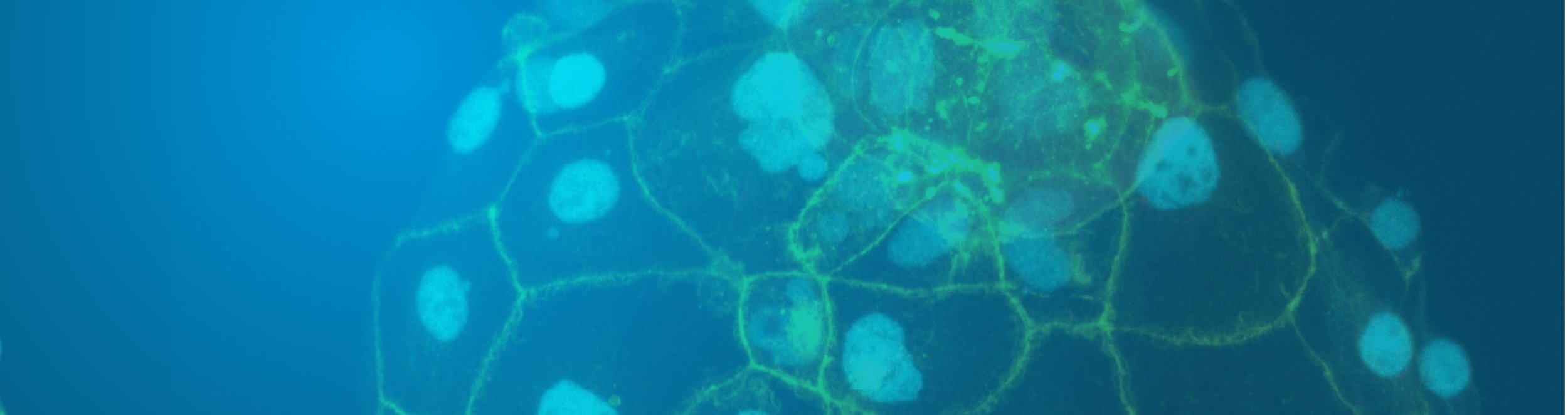
Background image: Early human embryo model from stem cells in culture (Image by Matteo Molè, Rugg-Gunn lab)
Identifying when and how a single fertilised egg cell generates the major cell types that will eventually become tissues and organs is fundamental to understand healthy human development as well as providing clues about disorders that occur during development and ways to improve the outcomes of fertility treatments.
Our work will focus on:
Tracking cells and studying cellular processes in the early human embryo to study when and how they become specific cell types
Investigating the molecular factors underlying early human embryo development
Creating models of early human development with stem cells, to investigate the genesis of primordial germ cells, the precursors of all the subsequent generations
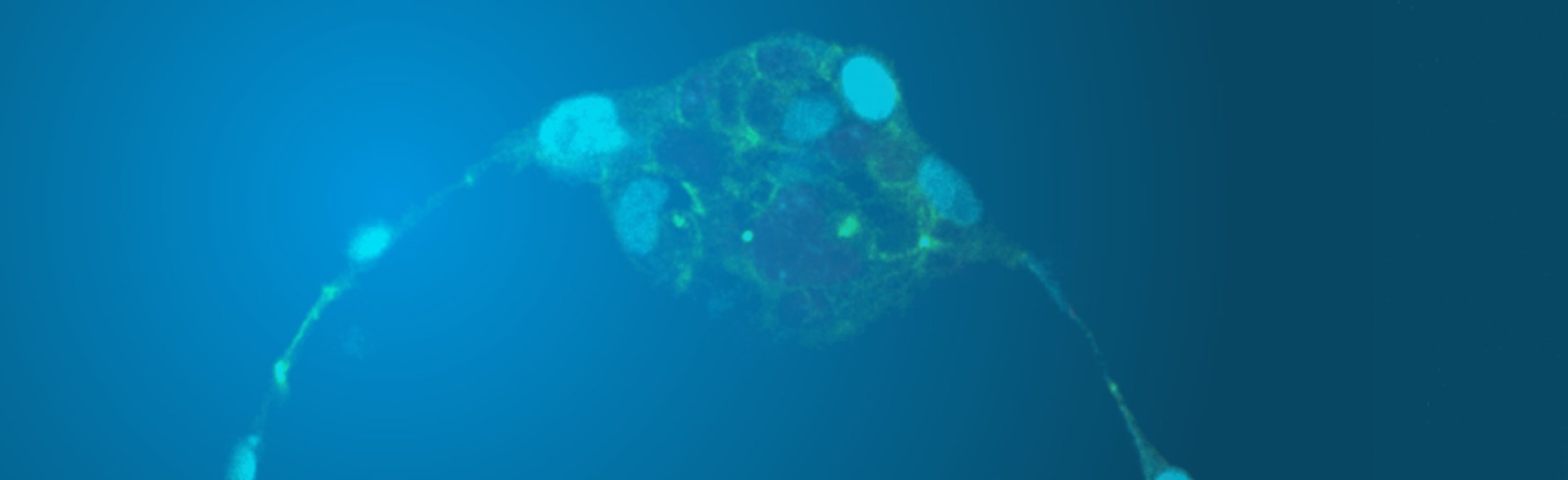
Human embryo at 6 days of development (Image from the Niakan lab)
Understanding early cell lineage decisions in human embryos is of fundamental biological importance and has significant clinical implications.
Background image: Cross section of an early human embryo model from stem cells in culture (Image by Matteo Molè, Rugg-Gunn lab)
We will develop innovative technologies to uncover the timing and mechanisms of human epiblast specification from the earliest stages of development until the emergence of the primary germ layers and of primordial germ cells.
To achieve this, we will work on:
Generating human embryos expressing fluorescent lineage markers, targeting the endogenous locus of genes involved in the epiblast, trophectoderm, or hypoblast specification to study the dynamics of lineage specification in living human embryos
Maximising the efficiency of CRISPR-mediated fluorescent protein integration to generate human reporter embryos
Investigating the epigenetic mechanisms that control early human embryo development and lineage specification by using a combination of multi-omics approaches (including parallel nucleosome, methylation and transcriptome sequencing) with an advanced in vitro culture system
Developing three-dimensional embryonic organoids using human pluripotent stem cells, representing early post-implantation human development up to and beyond the Carnegie stage (CS)7 gastrulation phase
Members
-

Prof Kathy Niakan
We investigate the mechanisms that direct ‘cell fate’ in human embryos and stem cells. This means studying the different factors that tell embryonic cells which type of cell to become.
-

Prof Mary Herbert
We aim to advance knowledge in the biology of germ cells and early embryos with a view of (i) improving the treatment of and understanding a major cause of infertility (ii) extending the scope of reproductive technologies to prevent transmission of disease.
-

Dr Peter Rugg-Gunn
We focus on understanding the epigenetic processes that are involved in regulating stem cells and the early stages of human development.

-

Prof Azim Surani
Gurdon Institute, University of Cambridge
We investigate the origin of primordial germ cells (PGCs), the precursors of gametes, in the context of early gastrulating human embryos leading to germline-soma segregation, using in vitro models generated from stem cells and ex vivo PGCs.
-

Dr Gavin Kelsey
We examine how epigenetic states are set up in oocytes – or egg cells – and influence gene expression in the embryo.
-

Dr Ahmed Abdelbaki
(Niakan lab)
-

Dr Magomet Aushev
(Herbert lab)
-

Dr Yuko Takeda
(Herbert lab)
-

Dr Yang Wang
(Rugg-Gunn lab)
-

Dr Jitesh Neupane
(Surani lab)
-
Former members
Dr Afshan McCarthy
Dr Matteo Molè

